Is Sugar a Strong or Weak Electrolyte
Weak bases and weak acids are considered weak electrolytes. Strong acids and strong bases are strong electrolytes eg HClaq H 2 SO 4 aq HClO 4 aq.

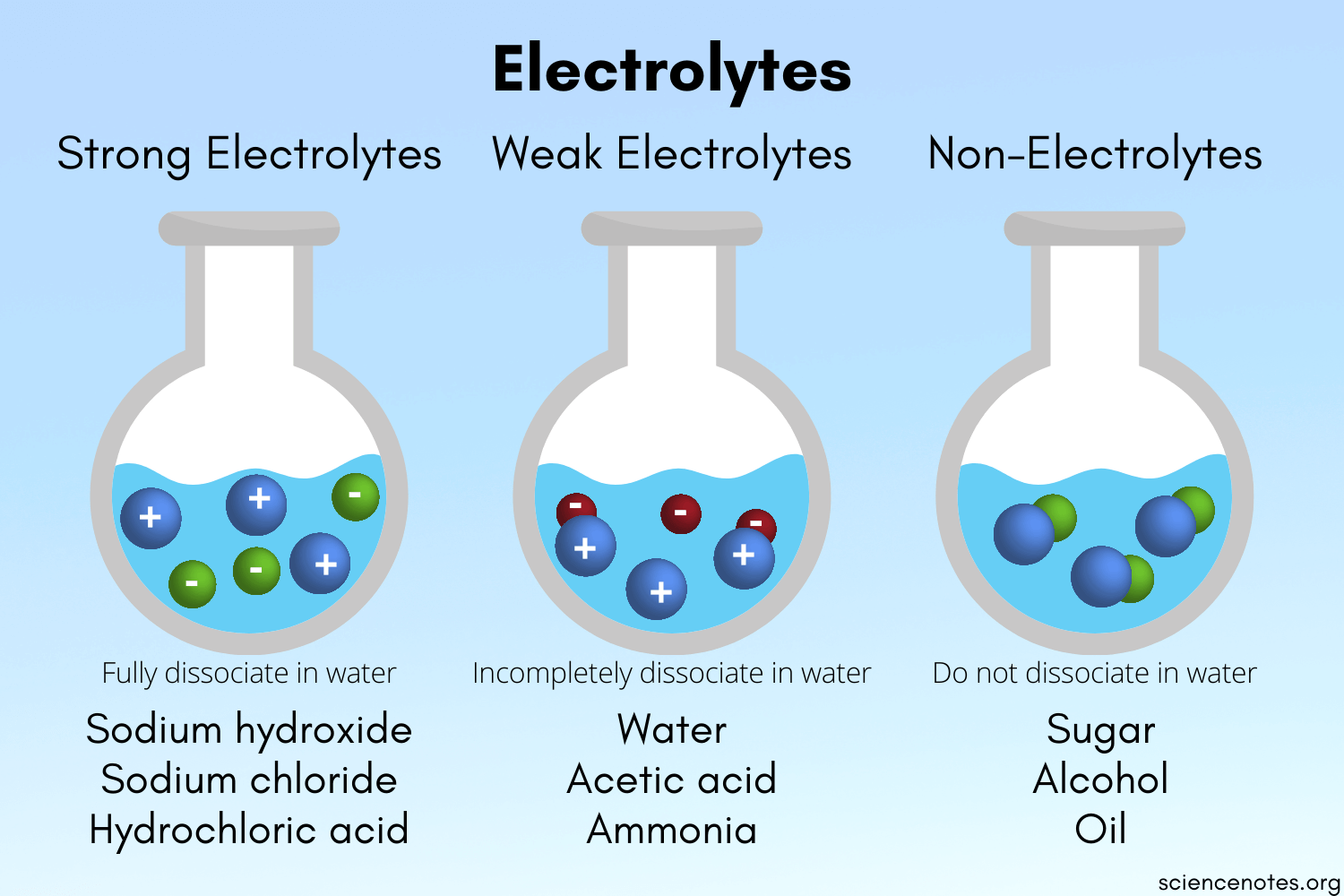
Electrolytes Definition Overview Expii
Weak electrolyte strong acid strong electrolyte weak acid weak electrolyte weak acid strong electrolyte strong acid nonelectrolyte.
. Learn with flashcards games and more for free. Acetic acid CH 3 COOH. Sugar for example dissolves readily in water but remains in the water as molecules not as ions.
Strong bases strong acids and salts are considered strong electrolytes. Salt is considered a strong electrolyte even though it has low solubility in the water because whatever the amount it dissolves in the water is completely ionised. Hence there will be only ONE particle when sugar is added to water.
Sugar is classified as a non-electrolyte. This means when dissolved in water it will not dissociate into ions. Similarly is acetic acid a strong electrolyte.
NH 3 - ammonia. As the name acetic acid suggests this substance is also an acid as well as a weak electrolyte. Strongweak electrolyte nonelectrolyte insoluble.
Molecular Compound Electrolyte Type Species in Solution sucrose table sugar non-electrolyte molecules only acetic acid HC 2 H 3 O 2 HOAc weak electrolyte molecules and some ions hydrogen chloride HCl strong electrolyte ions only 3. Question 13 1 point Identify sugar. The most familiar example of a strong electrolyte is table salt sodium chloride.
Sugar water is not an electrolyte. Sugar for example dissolves readily in water but remains in the water as molecules not as ions. 100 1 rating When sugar is dissolved in water it is soluble but it donot ionise into positive and negative ion so it is not.
When sugar dissolves in water it is described as A weak electrolyte B nonelectrolyte strong electrolyte When CH3COOH acetic acid dissolves in water it is described as. Acetic acid is considered a weak acid. And nitric acid HNO3 is a strong electrolyte.
There are virtually no. Weak electrolytes include weak acids weak bases and a variety of other compounds. Strong acids and strong bases are strong electrolytes eg HClaq H 2 SO 4 aq HClO 4 aq.
Because C is a non-met. Sugar is a non-electrolyte. Because Li is a meta.
Sucrose C12H22O11 is a nonelectrolyte. Strong electrolytes are substances that completely break apart into ions when dissolved. Hypochlorous acid HClO is a weak electrolyte.
Sugar is a non-electrolyte when dissolve in water View the full answer Transcribed image text. Acetic acid is a weak acid so it is a weak electrolyte So a strong electrolyte do not completely ionize in weak electrolyte so the ionization is less and weak. The most familiar example of a strong electrolyte is table salt sodium chloride.
Strong electrolytes are substances that completely break apart into ions when dissolved. Identify table sugar which is sucrose C12H22O11. H 2 O - water weakly dissociates in itself.
HF is a Weak Electrolyte Common weak electrolyte are CH3COOH HNO2 NH3 H20 Is sugar water an electrolyte or non electrolyte. We therefore make a distinction between strong electrolytes such as sodium chloride and acetic acid which is an example of a weak electrolyte. Sugar is classified as a non-electrolyte.
Strong electrolyte strong acid nonelectrolyte weak electrolyte strong acid weak electrolyte weak acid strong electrolyte weak acid. Acetic acid is the. Strong acids react completely with base to form salts and release protons into solution while weak acids do not react with base under normal conditions.
HF - hydrofluoric acid. Accordingly we classify acetic acid as a weak acid. Weak electrolyte strong acid strong electrolyte weak acid weak electrolyte weak acid strong electrolyte strong acid.
The pKa value of acetic acid is 48. Molecular Compound Electrolyte Type Species in Solution sucrose table sugar non-electrolyte molecules only acetic acid HC 2 H 3 O 2 HOAc weak electrolyte molecules and some ions hydrogen chloride HCl strong electrolyte ions only 3. Most compounds that contain nitrogen are weak electrolytes.
CH 3 CO 2 H - acetic acid. Weak electrolytes only partially break into ions in water. In chemistry classes acids are often discussed in terms of their pKa values.
View the full answer. To tell if C12H22O11 Sucrose or Table Sugar is an electrolyte or nonelectrolyte we first need to know what type of compound we have. There are virtually no.
To tell if To tell if LiCl Lithium chloride is an electrolyte or non-electrolyte we first need to know what type of compound we have.
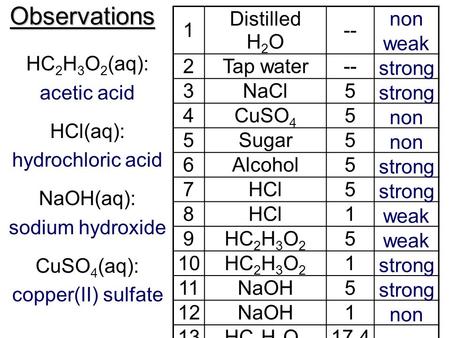
Weak Strong And Nonelectrolytes Ppt Video Online Download

Solved Match Each Compound With The Type Of Electrolyte It Chegg Com

Strong And Weak Electrolytes Study Guide Inspirit
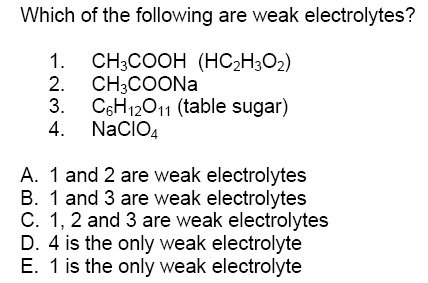
Solved Which Of The Following Are Weak Electrolytes Chegg Com

Is C12h22o11 Sucrose Or Table Sugar An Electrolyte Or Non Electrolyte Youtube

Solved Question 13 1 Point Identify Sugar Strong Chegg Com

Electrolytes And Nonelectrolytes Ppt Download

Solved Identify Sugar O Strong Electrolyte Strong Acid Chegg Com
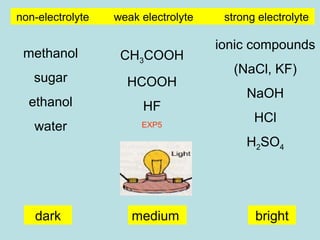
No comments for "Is Sugar a Strong or Weak Electrolyte"
Post a Comment